We focus on developing human models of neuroinflammation and education of trainees in the field of regenerative neuroimmunology. We have contributed 1) to the understanding mechanisms of migration of neural stem cells (NSCs) to areas of inflammation (Proc Natl Acad Sci U S A. 2004.). 2) The intrinsic capacity of NSCs to respond to immune cytokines and being target in inflammation. 3) In vivo effects of inflammation on NSCs niches, Oligodendrocyte progenitors cells and lack of remyelination in animal models of Multiple Sclerosis. (Ann Neurol. 2011 May;69(5):878-91).
|
Impact of immunity on NSCs/OPCs programs in models of MS.
Our studies demonstrated the NSC, OPCs and neurons harbours an intrinsic inducible program of inflammation that leads to changes in self-renewal and differentiation. Our lab studies the effects of inflammation on mouse and human NSCs to find pathways to prevent transcriptional changes in NSCs, OPCs and neurons. Front Cell Neurosci. 2023 Aug 16;17:1156802 J Neuroimmunol. 2019 Jun 15;331:1-3. human cellular models of cytokine-induced neurodegeneration
There is a lack of human-based translational models for neurodegenerative diseases. . Our lab studies the effects of inflammation on human-derived in vitro induced pluripotent stem cell (iPSC) and organoids. Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):8646-8648 Curr Protoc. 2021 Feb;1(2):e15 |
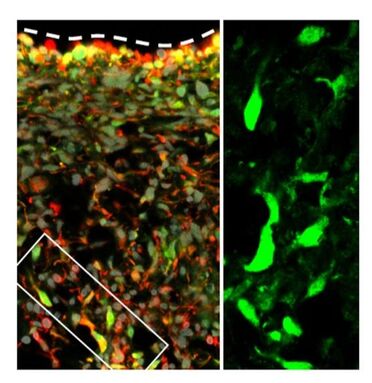
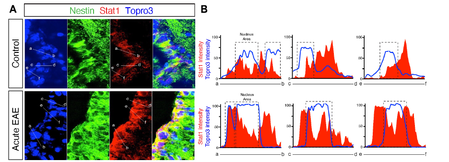 Confocal imaging of the SVZ during EAE upregulating STAT1, an inducible transcription factor that mediates the effect of Th1 cytokine IFN-g in NSCs. Confocal imaging of the SVZ during EAE upregulating STAT1, an inducible transcription factor that mediates the effect of Th1 cytokine IFN-g in NSCs.
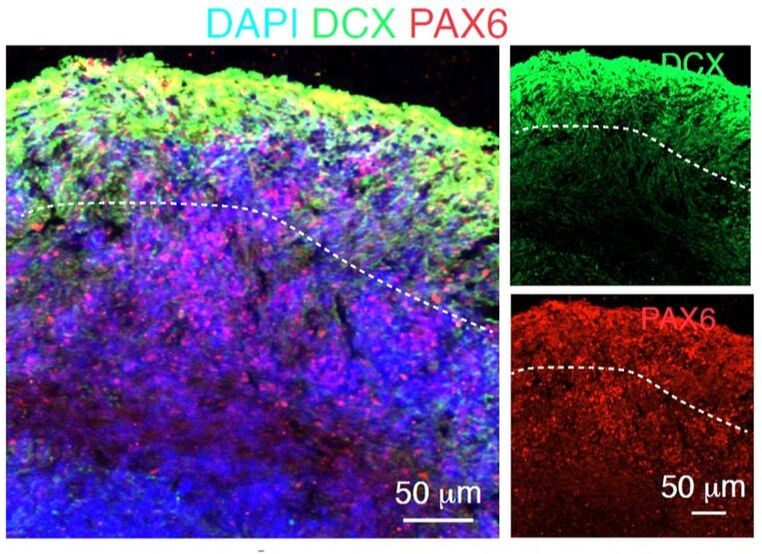 Confocal imaging of radial glia transcription factor PAX6 and the newborn neurons marker (DCX) doublecortin in human cerebral organoids Confocal imaging of radial glia transcription factor PAX6 and the newborn neurons marker (DCX) doublecortin in human cerebral organoids
|
|
Synthetic organogenesis of Glioblastoma stem cells and early immune interactions
Glioma stem cells (GSC) responses to an immune micro-environment, we recently showed a intrinsic immune program, we aim to define novel immune targets on GSCs using organoids. PNAS Nexus, 2024 Feb 2;3(2):pgae051 |
Clinical real-world observational MS research
We are interested in how social determinants and environmental exposures operate as catalysts to accelerate progression in MS. As such, the lab has embarked in the last four years in efforts to investigate social determinants in at risk populations.
We are interested in how social determinants and environmental exposures operate as catalysts to accelerate progression in MS. As such, the lab has embarked in the last four years in efforts to investigate social determinants in at risk populations.
|
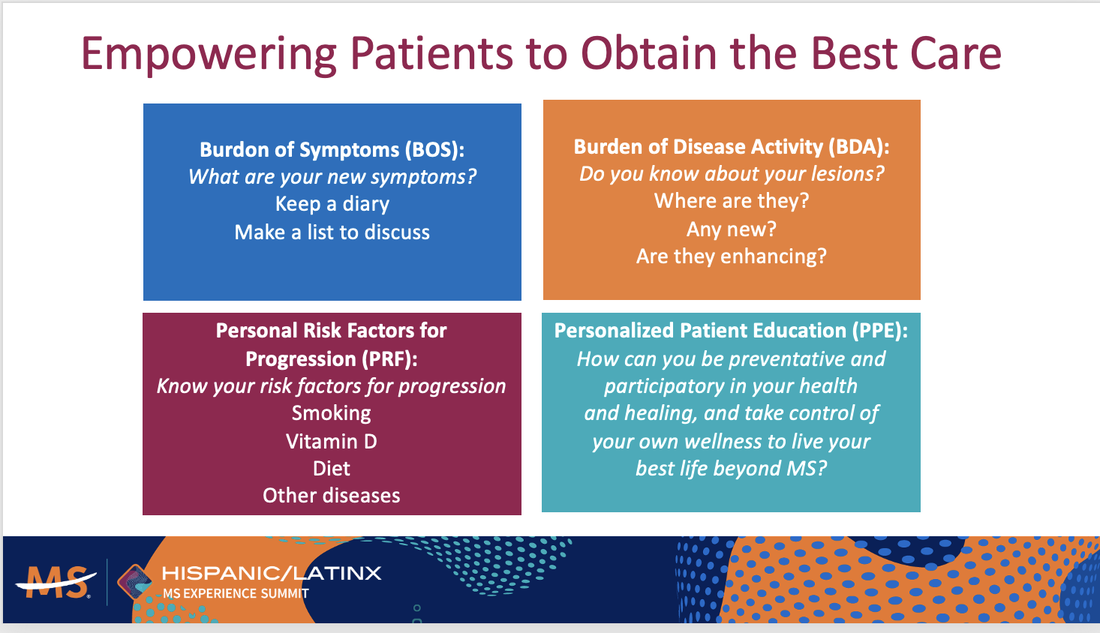
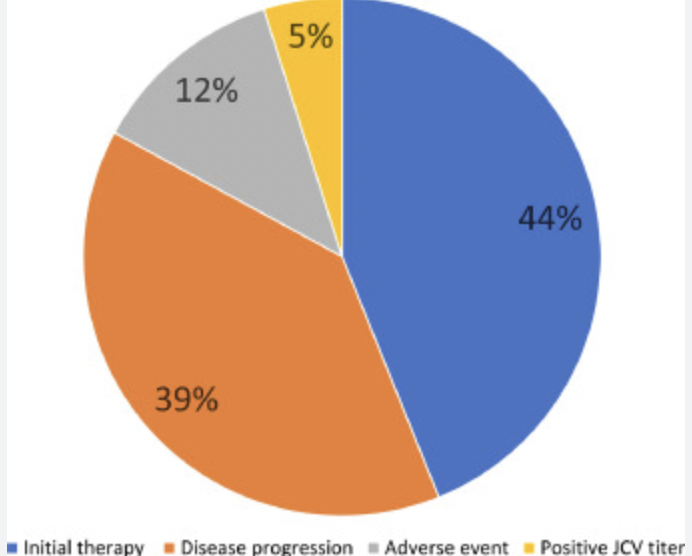
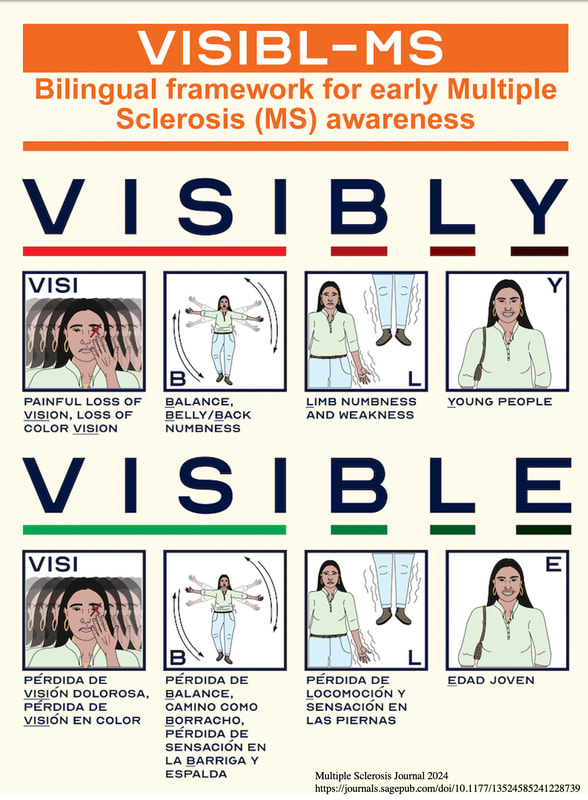
1) We created and directed the first AAN annual meeting course for diagnosis and treatment of MS in diverse populations in 2023 and 2024. 2) We created an educational tool, “the 4-square MS educational matrix” to help neurology residents, general neurologists, and patients learn about the modern proactive goals of care. Coban et al, 2021 Mult Scler Relat Disord. 2021 Jan;47:102631 (PMID: 33296855). 3) Our team defined the real-world impact of Ocrelizumab initiation in a diverse population. Coban et al, 2021 Mult Scler Relat Disord. 2021 Aug;53:103021.(PMID: 34077828). 4) Our team investigated new ways to decrease barriers to early diagnosis by utilizing DIR imaging in MS. Patel et al, 2023 (PMID: 36978252). J Neuroimaging. 2023 Jul-Aug;33(4):521-526. 5. Our team developed a novel tools to increase early awareness of MS. Please read our latest contribution published in MSJ, 2024 VISIBL-MS: A bilingual educational framework to increase awareness of early multiple sclerosis, Patel et al, MSJ 2024 https://journals.sagepub.com/doi/10.1177/13524585241228739 |
Annual Meeting Course for MS in Diverse population in 2023
"4-square education matrix" to educate patients and trainees about the NEDA visit and goals of care in MS.
First real-world Ocrelizumab initiation study in diverse population in the US.
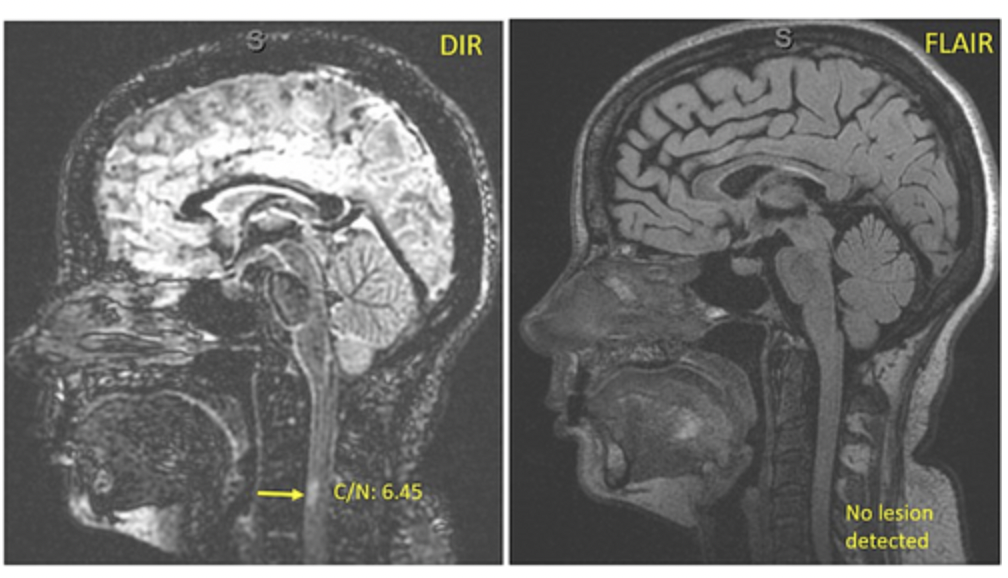
Study demonstrating the DIR-sequence in the brain is able to detect spinal cord lesions in MS patients
|